
A one-step immunoassay in which a chemically labeled drug anti-body (drug protein conjugate) impregnated on a porous test panel competes with the targeted drug or drug metabolite which may be present in the urine sample. Tests use the lateral flow, competing anti-body site technology identical to other forensic type tests using human urine as the testing medium.
The test device, generally a cassette, contains membrane strips which are pre-coated with drug-protein conjugate (or antibody) on the test band. The colored antibodies (or drug-protein conjugate)-colloidal gold conjugate pads are placed at the end of the membrane. In the absence of drug in the urine, the solution of colored antibody (or drug-protein conjugate)-colloidal gold conjugate and urine moves upward chromatographically by the capillary action across the membrane. This solution then migrates to the immobilized drug-protein antibody (or drug-protein conjugate)-colloidal gold conjugate then attaches to the drug-protein conjugate (or antibody) to form a visible line as the antibody complexes with the drug conjugate. Therefore, the formation of a visible precipitant in the test zone occurs when the test urine is negative for the drug. When drug is present in the urine, the drug/metabolite antigen competes with drug-protein conjugate on the test band region for the limited antibody. When a sufficient concentration of drug is present, it will fill the limited antibody binding sites. This will prevent attachment of the colored antibody (or drug-protein conjugate)-colloidal gold conjugate to the drug-protein conjugate (or antibody) zone on the test band region. Therefore, absence of the color band on the test region indicates a positive result.
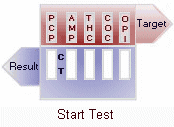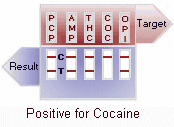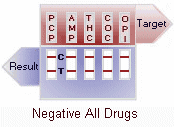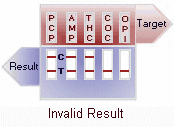
A control band that has a different antigen/antibody reaction is added to the immunochromatographic membrane strip at the control region (C) to indicate that the test has performed properly. This control line should always appear regardless of the presence of drug or metabolite. This means that negative urine will produce two colored bands and positive urine will produce only one band. Detailed graphic
The test kit should be stored at refrigerated or at room temperature or 2-30° C (36-86° F) in the sealed pouch for the duration of the shelf-life. In general our test kits have a shelf-life exceeding 12 to 14 months. For details on how to use the QuickScreen Rapid drug tests, please refer to Drug Test Use