

The QuickScreen Pro Multi-Drug Test Card now incorporates an automatic timing device. The timer has two indicator "lights" that will turn a red color tone as critical periods are reached during the development of test panel results. A "result ready" indicator will show when test results are completely developed and ready to be interpreted, while a "result expired" indicator will show when test development time is beyond that specified for a correct interpretation of test results. This new timing device eliminates the need for a separate timer during testing and potential interpretation errors due to improper waiting periods.
The QuickScreen Pro Multi-Drug Test Card is formulated for use with human urine specimens only. Fresh urine does not require any special handling or pretreatment. Urine samples should be collected such that testing may be performed as soon as possible after the specimen collection, preferably during the same day. The specimen may be refrigerated at 2-8° C for 2 days or frozen at -20° C for a longer period of time. Specimens that have been refrigerated must be equilibrated to room temperature prior to testing. Specimens previously frozen must be thawed, equilibrated to room temperature, and mixed thoroughly prior to testing.
Urine specimens may be potentially infectious. Proper handling and disposal methods should be established. Avoid cross-contamination of urine samples by using a new specimen collection container for each urine sample.
Note: Urine specimens, and all materials coming in contact with them, should be handled and disposed as if capable of transmitting infection. Avoid contact with skin by wearing gloves and proper attire.


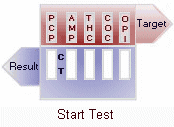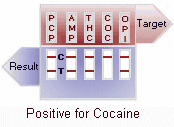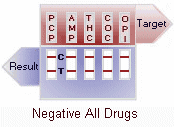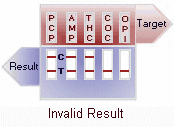
(-) Negative: Two colored lines adjacent to each drug name should be observed in the viewing window. The line in the test region (T) is the drug probe line; the line in the control region (C) is the control line, which is used to indicate proper performance of the device. The test line intensity may be weaker or stronger than that of the control line.
(+) Positive: Only one colored line appears in the control region (C). The absence of a test line indicates a positive result for that drug.
(-/+) Inconclusive: A faint*, indistinguishable line appears in the test region (T) . While probably suggesting a negative result, the test is inconclusive and suggests that the drug in the panel could be near the cut-off concentration level for detection. Perform a second test or take the sample for more extensive laboratory testing. Because of the quality controls built into the test device, an inconclusive result is highly unlikely.
Invalid: No line appears in the control region. Under no circumstances should a positive sample be identified until the control (C) line forms in the viewing area. If the control line (C) does not form, the test result is invalid and the assay should be repeated. View Result Graphic

*Note: An extremely faint line (barely visible) in the test region indicates that a drug metabolite in the sample may near the cut-off concentration level for the test. Normally, any line in the test area should be interpreted as a negative test. If suspicion or uncertainty remains regarding the test results, there are several options available: The donor can be retested at another time with a new test card and sample, the same sample can be retested using a new test card, or the sample can be evaluated by a testing laboratory.
For details on the chemistry technology of the test please refer to test technology or the Drug FAQ page.